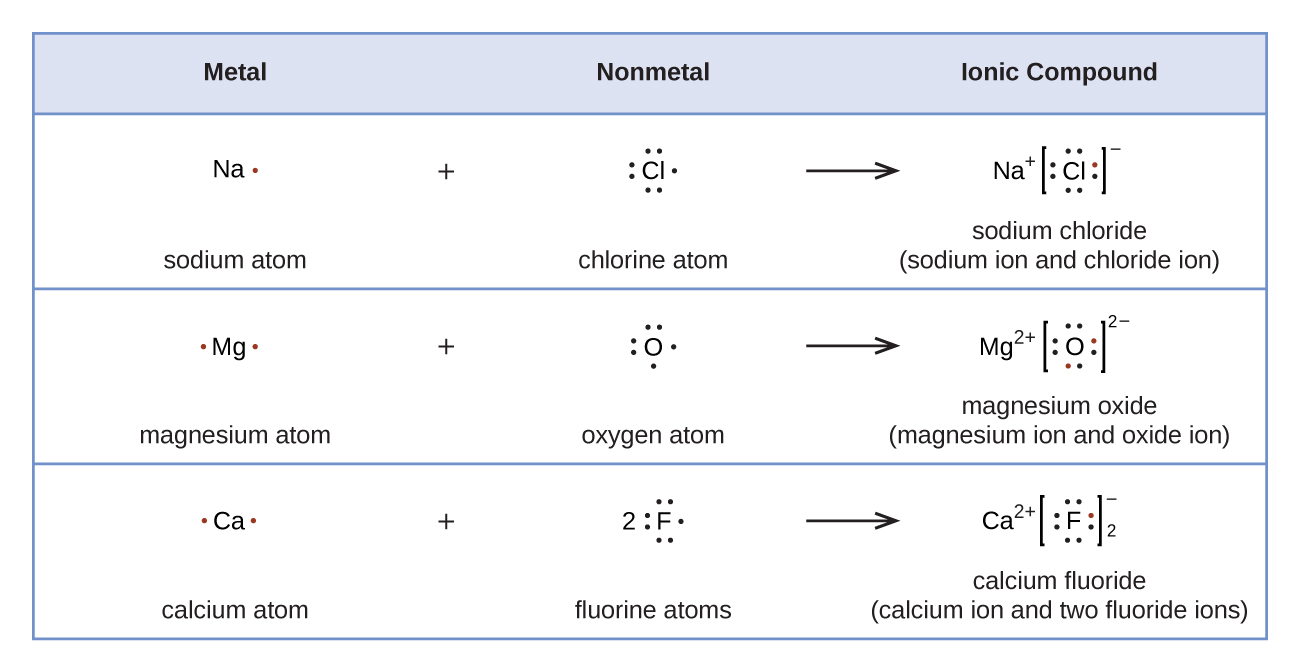
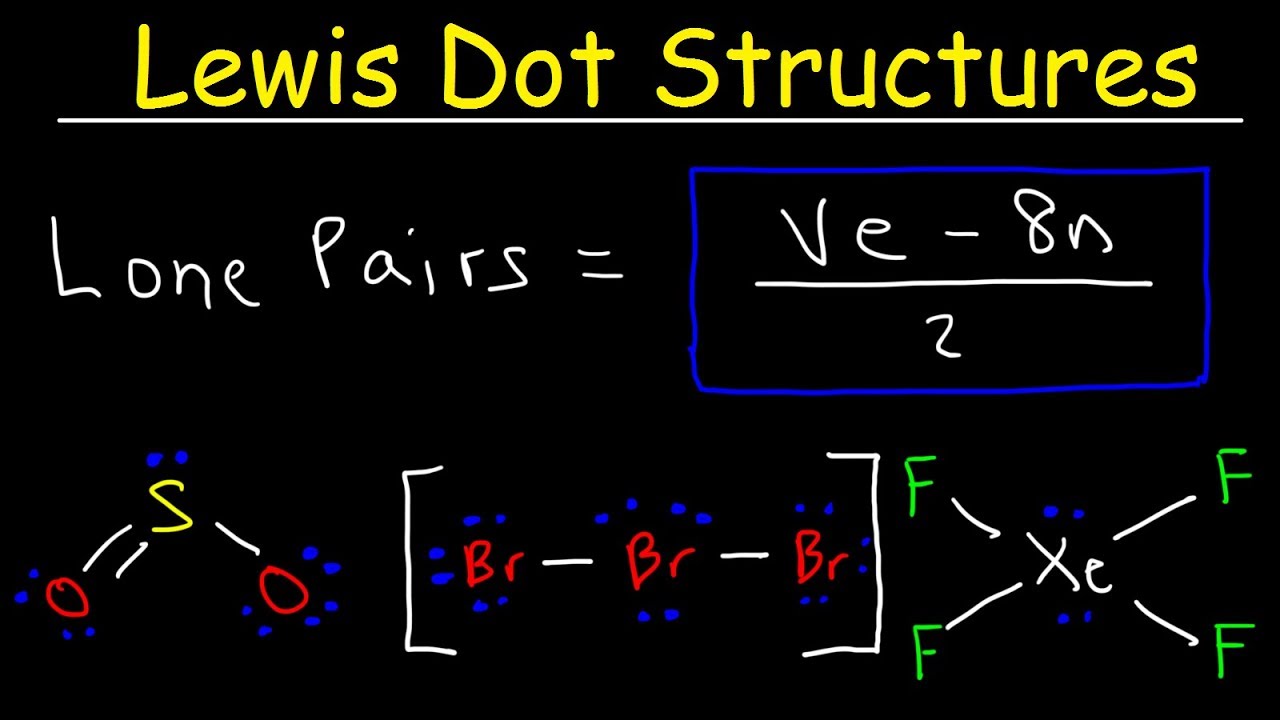
He described what he called the cubical atom, because a cube has 8 corners, to represent the outer valence shell electrons which can be shared to create a bond. This was his octet rule. Rules for drawing Lewis dot structures. Count the number of valence e-each atom brings into the molecule. For ions, the charge must be taken into account. . Lewis structures incorporate an atom’s formal charge, which is the charge on an atom in a molecule, assuming that electrons in a chemical bond are shared equally between atoms. Lewis dot diagrams are often employed to visualize the covalent bonding between atoms in a compound.
Formal charge:
Let us draw the Lewis structure for carbon dioxide.
1. Skeletal structure
2. Total number of valence electrons in CO2
=[1 x 4(carbon)] +[2 x 6(oxygen)] = 4+ 12 = 16
3. Draw single bonds between atoms. Two bonds can be drawn as shown in the figure for CO2 which accounts for four electrons (2 bond pairs).
4. Distribute the remaining twelve electrons (16 - 4= 12) as six lone pairs starting from most electronegative atom, the oxygen. Six lone pairs are distributed to the two terminal oxygens (three each) to satisfy their octet.
5. Verify weather all the atoms have octet configuration. In the above distribution, the central carbon has two pair short for octet. Therefore, to satisfy the octet rule two lone pairs from one oxygen or one pair from each oxygen can be moved to form multiple bonds, leading the formation of two possible structures for carbon dioxide as shown below
Similarly, the Lewis structure for many molecules drawn using the above steps gives more than one acceptable structure. Let us consider the above mentioned two structures of carbon dioxide.
Which one the above forms represents the best distribution of electrons in the molecule. To find an answer, we need to know the formal charge of each atom in the Lewis structures. Formal charge of an atom in a molecule, is the electrical charge difference between the valence electron in an isolated atom and the number of electrons assigned to that atom in the Lewis structure.
Where,
Nv- Number of valence electron of atom in its isolated state.
Nl - Number of electrons present as lone pairs around the atom in the Lewis structure

Nb - Number of electrons present in bonds around the atom (bond pairs) in the Lewis structure]
Now let us calculate the formal charge on all atoms in both structures,
For Structure 1,

For structure 2
Formal charge on carbon
After calculating the formal charges, the best representation of Lewis structure can be selected by using following guidelines.
1. A structure in which all formal charges are zero preferred over the one with charges.
2. A structure with small formal charges is preferred over the one with higher formal charges.
3. A structure in which negative formal charges are placed on the most electronegative atom is preferred.
In case of CO2 structures, the structure one is preferred over the structure 2 as it has zero formal charges for all atoms.
Formal charge:
Let us draw the Lewis structure for carbon dioxide.
1. Skeletal structure
2. Total number of valence electrons in CO2
=[1 x 4(carbon)] +[2 x 6(oxygen)] = 4+ 12 = 16
Covalent Bonds Lewis Dot Diagram
3. Draw single bonds between atoms. Two bonds can be drawn as shown in the figure for CO2 which accounts for four electrons (2 bond pairs).
4. Distribute the remaining twelve electrons (16 - 4= 12) as six lone pairs starting from most electronegative atom, the oxygen. Six lone pairs are distributed to the two terminal oxygens (three each) to satisfy their octet.
5. Verify weather all the atoms have octet configuration. In the above distribution, the central carbon has two pair short for octet. Therefore, to satisfy the octet rule two lone pairs from one oxygen or one pair from each oxygen can be moved to form multiple bonds, leading the formation of two possible structures for carbon dioxide as shown below
Similarly, the Lewis structure for many molecules drawn using the above steps gives more than one acceptable structure. Let us consider the above mentioned two structures of carbon dioxide.
Which one the above forms represents the best distribution of electrons in the molecule. To find an answer, we need to know the formal charge of each atom in the Lewis structures. Formal charge of an atom in a molecule, is the electrical charge difference between the valence electron in an isolated atom and the number of electrons assigned to that atom in the Lewis structure.
Where,
Nv- Number of valence electron of atom in its isolated state.
Nl - Number of electrons present as lone pairs around the atom in the Lewis structure
Nb - Number of electrons present in bonds around the atom (bond pairs) in the Lewis structure]
Now let us calculate the formal charge on all atoms in both structures,
For Structure 1,
Lewis Structure Covalent Compound Calculator
For structure 2
Formal charge on carbon
After calculating the formal charges, the best representation of Lewis structure can be selected by using following guidelines.
1. A structure in which all formal charges are zero preferred over the one with charges.
2. A structure with small formal charges is preferred over the one with higher formal charges.
3. A structure in which negative formal charges are placed on the most electronegative atom is preferred.
In case of CO2 structures, the structure one is preferred over the structure 2 as it has zero formal charges for all atoms.